Periodic Classification of Elements is one of the important chapters of Chemistry in Class 10. Mostly students find it difficult to understand the concepts of Period Classification of Elements, Periodic Tables like Modern Periodic Table, Mendeleev’s Periodic Table and properties of elements of Periodic Table.
Students cannot skip this chapter as Periodic Classification of Elements is a very scoring and an important chapter for Class 10 NCERT Exam. Students need not worry about it anymore as they can study the concepts of Periodic Classification of Elements from here. Students can find the Periodic Classification of Elements Class 10 notes along with the important points, question, and solutions from here.
Periodic Classification of Elements Topics
Students studying in Class 10 looking for Periodic Classification of Elements notes can find it here. The important topics of the Periodic table of elements are explained below. In this article, we will discuss the following concepts of the Periodic table of elements:
- Classification of Elements
- Dobereiner’s Triads
- Newlands Law of Octaves
- Mendeleev’s Periodic Table
- Modern Periodic Table
- Trends in Modern Periodic Table
- Some Important Questions & Answers
Periodic Table of Elements - Classification
The Classification means identifying similar species and grouping them together. These groups are defined as metals, metalloids, nonmetals, and inert gases etc.
Here is the Periodic table of elements.

Dobereiner’s Triads
Dobereiner classified elements on the basis of Law of Triads. The law of triads states that the atomic mass of the middle element of a triad is almost the arithmetic mean of the other two elements. According to Johann Wolfgang Döbereiner, when three elements in a triad were written in the order of increasing atomic masses, then the atomic masses in the middle element was roughly the average of the atomic masses of the other two elements.
|
Elements of Periodic Table |
Atomic Mass |
Arithmetic Mean |
|
Lithium Sodium Potassium |
7 23 39 |
7+39 = 23 2 |
|
Chlorine Bromine Iodine |
35.5 80 126.5 |
35.5+126.5 = 80 2 |
|
Calcium Strontium Barium |
40 87 137 |
37+40 = 8 2 |
Drawbacks of Dobereiner’s triads
However, there are some drawbacks of Dobereiner’s triads as well. Such drawbacks are like he could identify only a few triads so the law could not gain importance. In the triad of Fe, Co, and Ni, all the three elements have a nearly equal atomic mass and thus it doesn’t follow the above law.
Newlands Law of Octaves
According to the Newlands law of Octaves, the elements are arranged in such way that the eight elements starting from a given one has the properties which are the repetition of those of the first if arranged in the increasing order of the atomic weight. It is like the eight note of music scale.
|
Newland’s Arranged Elements in Octaves |
||||||
|
sa (do) |
Re (re) |
Ga (mi) |
Ma (fa) |
Pa (so) |
Da (la) |
Ni (ti) |
|
H |
Li |
Be |
B |
C |
N |
O |
|
F |
Na |
Mg |
Al |
Si |
P |
S |
|
Cl |
K |
Ca |
Cr |
Ti |
Mn |
Fe |
|
Co and Ni |
Cu |
Zn |
Y |
In |
As |
Se |
|
Br |
Rb |
Sr |
Ce and La |
Zr |
- |
- |
Drawbacks of Newland Law of Octaves
The drawbacks of Newland Law of Octaves are:
- It was found that the law of Octaves was applicable up to only one calcium because, after calcium, every eight elements did not possess properties similar to that of the first.
- Later on, several new elements were discovered but their properties did not fit into the Law of Octaves.
Mendeleev’s Periodic Table - Periodic Classification of Elements
Mendeleev arranged elements based on their atomic masses. He arranged 63 elements which are known at that time in the periodic table. He also observed that when the elements were arranged in increasing order of their atomic masses, then there was a periodic recurrence in their physical and as well as chemical properties. Hence, Mendeleev created periodic law. The law states that the properties of elements are the periodic function of their atomic masses. In the Mendeleev’ periodic table, the vertical columns are called groups and horizontal rows are called periods.
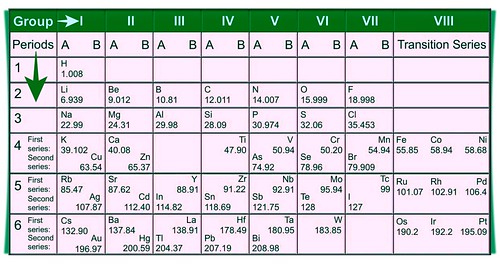
Mendeleev’s periodic table was found to be consistent with the observations made about the physical as well as chemical properties of the elements.
Mendeleev left some gaps in his Periodic Table in order to recognize the existence of some elements that had not been discovered. For example, gallium, scandium, and germanium were discovered later and they have properties similar to Eka - Boron, Eka – Aluminum, and Eka - Silicon, respectively. The properties of Eka - Aluminum and Gallium are given in the table below:
|
Periodic table Property |
Eka - Aluminum |
Gallium |
|
Atomic Mass |
68 |
69.7 |
|
Formula of Oxide |
E2O3 |
Ga2O3 |
|
Formula of Chloride |
ECl3 |
GaCl3 |
Modern Periodic Table - Periodic Classification of Elements
Henry Moseley proposed the modern periodic table. According to that table, the physical and chemical properties of the elements are the periodic function of their atomic number. He also showed that the atomic number of the element has the more fundamental properties than its atomic mass. In the modern periodic table, there are 18 vertical columns that are groups and 7 horizontal rows that are called periods.
Modern Periodic Table Properties and Trends
The trends observed in some important properties of the elements in moving down the group and in moving from left to right are given below:
Valency - The Valency can be defined as the combining capacity of the atom of an element in order to get the stable configuration. Like 8 electrons in valence shell and in some cases, there are 2 electrons. If we see from top to bottom in the group, the one shell keeps on getting added from one element to another.
Atomic Size - The atomic size refers to the distance between the centre of the nucleus of the isolated atom to its outermost shell which contains electrons. The atomic radius decreases while moving from left to right along a period. In a group, the atomic size decreases from top to bottom because of the increase in the number of shells.
Metallic and Non-Metallic Properties - In a period from left to right, the metallic character decreases and non-metallic character increases.
In a group from top to bottom, the metallic character increases and the non-metallic character decreases.
Electronegativity - The value of electronegativity increases in the period from left to right and decreases in the group from top to bottom.
|
Periodicity in Properties |
||
|
Properties |
Across a Period (Left to Right) |
Down the Group |
|
Valency |
First increases from 1 to 4 than Decreases from 4 to 0 |
Remains Same |
|
Atomic Size |
Decreases |
Increases |
|
Metallic Character |
Decreases |
Increases |
|
Non-Metallic Character |
Increases |
Decreases |
Periodic Classification of Elements Class 10 - Important Questions
After studying the Periodic Classification of Elements Class 10 notes, students should check important questions of the chapter. Studying important questions is very important as it helps to check our understanding of concepts and also helps to prepare for the exam. For students studying Periodic Classification of elements chapter, here are some important questions to practice for the exam. The Periodic Classification of Elements Questions in the PDF format below. Students are advised to download the PDF and attempt the questions to get a clarity of the concepts.
Download Periodic Classification of Elements Questions
Periodic Classification of Elements - Important Questions
Q - 1. Do Dobereiner’s triads also exist in the columns of Newland’s Octaves? Compare and find out.
Ans. Triads of Li, Na and K exist in Newland’s octaves.
- Triads of Cl, Br and I don’t exist as there Co and Ni come between Cl and Br.
- Triads of Ca, Sr and Ba don’t exist as there Zn comes between Ca and Sr.
Q - 2. An element has electronic configuration 2, 8 and 3. What is the atomic number of this element? To which to which (1) group and (2) period this element belongs?
Ans. The Atomic number is 13.
(1)Group 13 (2) Period 3
Q - 3. An element placed in the second group and third period of the of the periodic table burns in the presence of oxygen to form a basic oxide.
(1) Identify the element
(2) Write the electronic configuration
(3) Write the balanced equation when it burns in the presence of air
(4) Write the balanced equation when the oxide dissolves in the water
Ans. (1) Magnesium (Mg)
Ans. (2) K, L, and M - 2, 8, and 2
Ans. (3) 2Mg(s) + O2(g) 2MgO(s)
Ans. (4) MgO(s) + H2O(I) Mg(OH)2(aq)
Q - 4. Atomic numbers of a few elements are given below:
10, 20, 7, 14
(1) Identify the elements.
(2) Identify the group number of these numbers in the periodic table
(3) Identify the periods of these elements in the periodic table
(4) What would be the electronic configuration of each of these elements
(5) Determine the valency of these elements
Ans. (1) Elements – Ne (Neon), Ca (Calcium), N (Nitrogen), and Si (Silicon)
Ans. (2) Group – 18, 2, 15, and 14
Ans. (3) Period – 2, 4, 2, and 3
Ans. (4) Electronic Configuration – (2,8), (2,8, 8,2), (2,5), (2,8,4)
Ans. (5) Valency – 0,2,3,4
Q - 5. What are the drawbacks of Newland’s Law of Octaves?
Ans. The drawbacks of Newland’s Law are:
- It was not applicable throughout the arrangements as it was applicable up to calcium only. The properties of the elements listed after the calcium showed no resemblance to the properties of the elements above them.
- It did not recognize the transition elements.
- The position of hydrogen was not justified along with fluorine and chlorine.
Q - 6. What are the drawbacks of Dobereiner’s Classification?
Ans. The drawbacks of Dobereiner’s Classification are:
- The classification into triads left room for chance. It is possible to group quite dissimilar elements into the triad.
- A large number of elements can’t be grouped into the triad.
Q - 7. Name three elements that have a single electron in their outermost shell.
Ans. Lithium, Sodium, and Potassium
Q - 8. Name 2 elements that have 2 electrons in their outermost shell.
Ans. Magnesium and Calcium
Q - 9. Name 3 elements that have a single electron in their outermost shell
Ans. Helium, Neon, and Argon
Q - 10. Which element has two shells with the filled outermost shell?
Ans. The element is Neon; 2 (K) 8 (L).
In the case of any query related to Periodic Classification of Elements Class 10, write to us in the comment section below.










